HydroFLOW Technology Protects Your Equipment against Corrosion.
Current passing along a metal pipe creates a coaxial magnetic field, or “skin effect,” interfering with the electro-chemical reaction necessary for corrosion to take place. Introducing Hydropath Technology to a water system inhibits internal pipe corrosion by up to 65%.

Microbiologically Induced Corrosion
Microbiologically induced corrosion (MIC), is a mode of corrosion caused by microbes that reside in colonies (biofilm) or dispersed in water that cause the corrosion or influence other corrosion processes of metallic materials.
MIC is caused by bacterial microbes in combination with four other environmental conditions: metals (host location), nutrients, water, and are usually anaerobic. These bacteria are ubiquitous in the environment and metallic piping materials. When all of these environmental conditions are present, then microbial growth will occur.
When the nutrients in the system are consumed, the microbes may become dormant. When the environmental conditions, i.e. nutrients are replenished, the microbial growth resumes. Water lines that develop scale may coat colonies of microbes that serves to protect them from biocides, and certain species that use iron as a food source can rapidly degrade pipes resulting in numerous pinhole leaks and eventually degradation of a majority of the piping.
Contact Us to Schedule a Risk-Free Evaluation, or
To Learn More about HydroFLOW for corrosion protection.
The HydroFLOW™ Water Conditioner: an easily installed, eco-friendly device that protects equipment assets against corrosion and premature failure, extending their useful life.
Download Lab Reports and Case studies for Corrosion Protection on our Resources Page

Figure 1: A tube-in-shell heat exchanger shown before and after (Left/ Right) the application of the Hydropath signal, where not only has the limescale been removed but magnetite has begun to form as a hard black deposit.
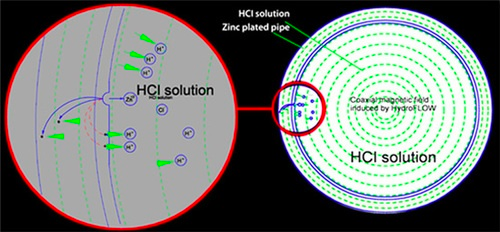
Figure 2: A diagram showing how the skin effect reduces corrosion on the inner surface of the pipe.
Based on over twenty-five years of experience, the patented HydroFLOW technology works with all equipment and piping systems, including:
- Cooling towers
- Hot Water Boilers
- Heat Exchangers
- Water and Wastewater Piping
- Commercial pools
- Wells: water, injection, oil and gas
- Pumps
Installed on the exterior of any piping system or pipe material, the HydroFLOW™ device employs a ferrite ring to induce an oscillating 150 kHz frequency that penetrates the pipe wall and travels both upstream and downstream from the point of installation. The water within the piping system, acts as a conduit to propagate the signal throughout the system, conditioning the water, whether it is moving or stationary. Testing has proven that the HydroFLOW™ signal is more efficient and effective than similar water conditioning technologies.
HydroFLOW technology protects piping systems and equipment due to the “skin effect” of the oscillating signal. The RF signal passing along a metal pipe creates a coaxial magnetic field, forcing free electrons to migrate to the exterior of a piping system (i.e., the “skin effect”), interfering with the electro-chemical chain reaction necessary for corrosion to occur. Within a carbon steel piping system, the signal creates a reducing environment, promoting the Formation of Magnetite in low temperature, oxygen-rich applications, such as cooling towers. As a hard dense layer on the piping interior surface, the magnetite protects the carbon steel substrate from oxidation
Additionally, the HydroFLOW signal inhibits the growth of SRB colonies and Microbial Induced Corrosion (MIC). Slow-moving or standing waters are prone to the development of bacteria, particularly SRBs, since these areas are not well penetrated by disinfectant chemicals, and flow turbulence is not available to dislodge biofilm. The HydroFLOW signal is propagated throughout a piping system, regardless of the flow velocity, penetrating and protecting slow-moving or standing channels from bacterial growth, inhibiting and/or preventing MIC.
HydroFLOW units are available to fit pipe sizes ranging from 1.5” to 72” outside diameter.
Common corrosion problems with HydroFLOW™ solutions:
- Microbial Induced Corrosion (MIC). Slow-moving or standing waters are prone to the development of bacteria, particularly SRBs, since these areas are not well penetrated by disinfectant chemicals, and flow turbulence is not available to dislodge biofilm. The HydroFLOW signal is propagated throughout a piping system, regardless of the flow velocity, penetrating and protecting slow-moving or standing channels from MIC.
- Carbon steel corrosion: Like MIC, Slow-moving or standing waters are prone to corrosion since these areas are not well penetrated by corrosion inhibition chemicals. The HydroFLOW signal is propagated throughout a piping system, regardless of the flow velocity, penetrating and protecting slow-moving or standing channels from corrosion.




